Nonclinical Studies
Our nonclinical and toxicology team tests all components of the JUUL System including e-liquids, aerosols and device properties in order to assess the potential risk and chemical exposure to individual users. This includes measuring harmful and potentially harmful constituents (HPHCs) found in the smoke of combustible cigarettes — as outlined by the FDA — compared to the aerosol of the JUUL System. Our researchers also measure toxicological response of the JUUL System’s e-liquid and aerosols using in vitro assays.
Aerosol Toxicology Studies
Testing cigarette smoke and JUUL System aerosol for Harmful and Potentially Harmful Constituents
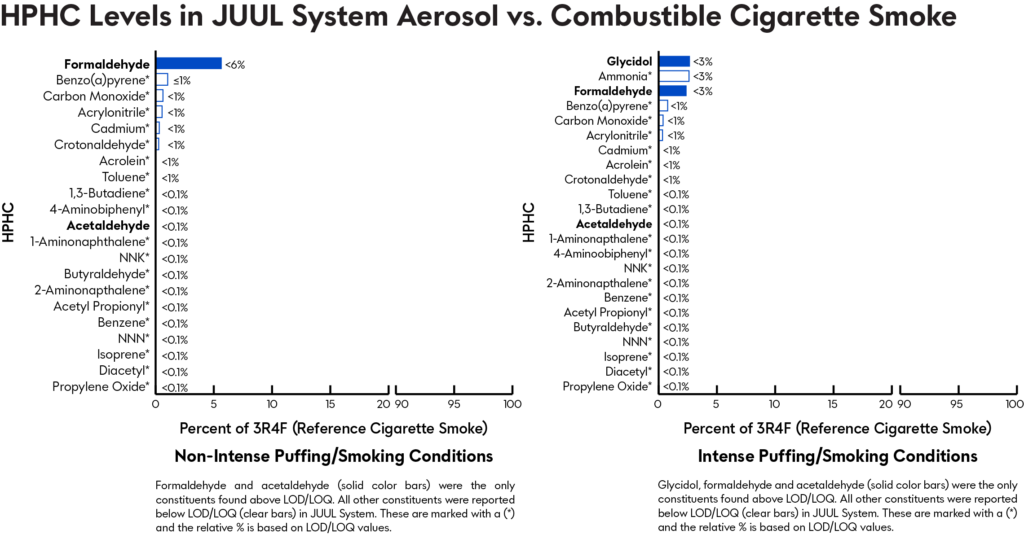
A study analyzed JUUL System aerosol samples with a 5.0% nicotine concentration for chemicals and HPHCs listed in the draft and final PMTA guidance for ENDS products. For those HPHCs with reported literature values from the 3R4F reference combustible cigarette, a comparison was performed.
Of the 26 constituents measured in both the JUUL System aerosol and combustible cigarette smoke, all constituents* in the JUUL System aerosols were present at lower levels relative to those in cigarette smoke, resulting in a 94% or greater reduction in aerosol levels of HPHCs or other measured chemicals.
*The only exceptions were propylene glycol, glycerin, and nicotine, which form the base formulation for JUUL System with benzoic acid.
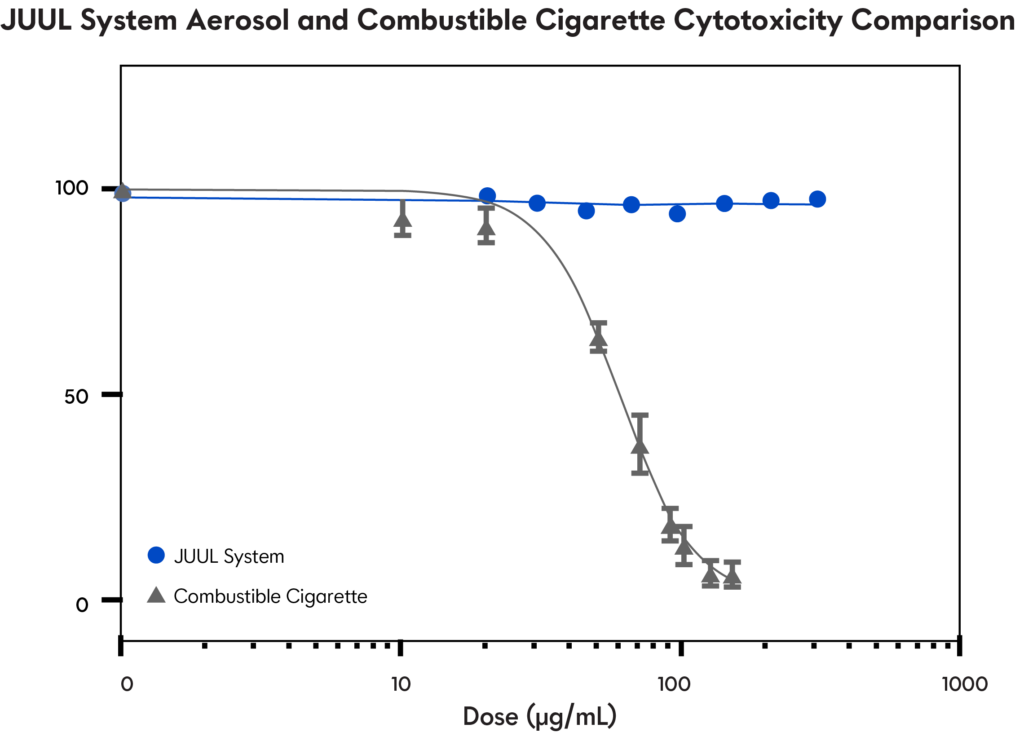
Cytotoxicity Studies
Testing the viability of cells exposed to JUUL System aerosol and cigarette smoke
In vitro studies have found that while combustible cigarette smoke caused cytotoxicity, JUUL System aerosol was non-cytotoxic, even at doses higher than the cigarette smoke.